Vanadium-chromium slag is a byproduct of metallurgical processes containing valuable metals like vanadium (V) and chromium (Cr), yet its improper disposal poses serious environmental and health risks. As industrial demands grow, the accumulation of this slag has become a pressing issue, driving researchers and industries to develop innovative recovery techniques. This article explores the origins of vanadium-chromium slag, its current disposal challenges, and the latest technologies transforming it from hazardous waste into a reusable resource. By understanding its production processes and recovery methods, we can unlock its potential while minimizing ecological harm.
What is Vanadium-Chromium Slag?
Vanadium-chromium slag is hazardous waste generated during smelting processes, containing valuable vanadium and chromium metals. Improper handling can cause severe environmental pollution, but recycling turns waste into treasure.
Vanadium-chromium slag is a byproduct from vanadium-iron and chromium-iron alloy production, composed mainly of vanadium pentoxide (V2O5) and chromium oxide (Cr2O3). This industrial residue contains valuable vanadium (worth $50-80/kg) alongside toxic hexavalent chromium contaminants requiring specialized treatment.
To understand the value and risks of vanadium-chromium slag, we must examine its origins and current management status. Let’s analyze three key questions.
Where Does Vanadium-Chromium Slag Come From?
You might not know that every step of stainless steel production can generate vanadium-chromium slag. These “industrial leftovers” are becoming key monitoring targets for environmental agencies.
Vanadium-chromium Slag Primarily Originates From Three Processes
- Vanadium-titanium magnetite smelting.
- Chromite ore processing for chromium-iron alloys.
- Secondary refining of stainless steel.
China produces 80% of such slag in the Panzhihua-Xichang vanadium-titanium magnetite region, generating over 2 million tons annually.
Primary Sources and Production Volume of Vanadium-Chromium Reduction Slag in China
Primary Sources | Capacity/t | Enterprise |
Sichuan Province | 15000-20000 | Panzhihua Iron & Steel Group |
North China Region | 10000-15000 | Chengde Vanadium & Titanium, Jianlong Steel |
Northeast China Region | 15000-20000 | Jinzhou Ferroalloy Group, Jianlong Steel |
Main Components of Vanadium-Chromium Slag
V | Cr | Fe | Si | Mg | Ca | Na | Mn | S | CI |
5.1 | 13.4 | 3.8 | 0.8 | 7.8 | 13.5 | 4.1 | |||
6.4 | 31.9 | 11.6 | 4.7 | 1.4 | 3.0 | 5.7 | 1.5 | 4.8 | |
6.3 | 17.7 | 1.1 | 5.3 | 1.1 | 6.4 | 11.9 | |||
1.6 | 14.3 | 0.3 | 12.0 | 0.2 | 1.4 | 12.0 | 4.1 | ||
2.7 | 30.2 | 3.0 | 3.5 | 2.4 | 1.5 | 2.8 | 1.0 | ||
5.6 | 23.5 | 14.6 | 11.9 | 1.6 | 0.7 | 0.6 | |||
18.2 | 15.1 | 4.6 | |||||||
23.9 | 33.2 | 0.2 | 0.5 | 0.2 | 1.5 | ||||
4.9 | 12.7 | 0.9 | 5.3 | <0.5 | 0.9 | 9.3 | 9.2 | ||
3.8 | 12.0 | 0.2 | 5.4 | 0.5 | <0.4 | ||||
4.4 | 19.9 | 0.3 | 6.0 | 9.2 | |||||
12.7 | 16.4 | 0.3 | 2.8 | 0.1 | 1.6 | 0.1 | 2.9 | 0.9 |
The vanadium content in vanadium-chromium reduction slag is significantly higher than that in primary ores such as vanadium-titanium magnetite and vanadium-bearing shale, making it an important secondary vanadium resource.
Vanadium-chromium reduction slag contains 3%–30% V, 10%–35% Cr, 0%–30% Fe, 0%–15% Si, 0%–15% S, and 0%–10% Na, along with trace amounts of Mg, Ca, Mn, Cl, and other elements.
Vanadium-Chromium Slag Production Process
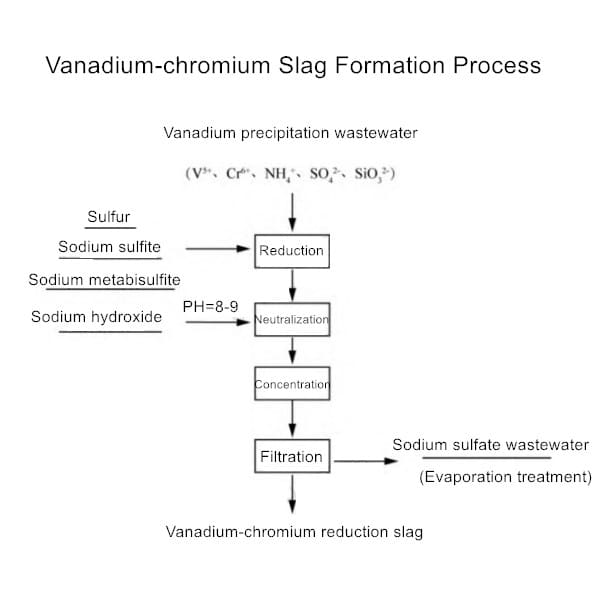
Slag Generation Process Explained
Primary Sources
1. Vanadium Production:
- Vanadium-titanium magnetite→Blast furnace→Hydrometallurgical extraction→Slag containing chromium
- Chemical reaction: 2FeV + 3O₂ → V₂O₅ + Fe₂O₃ + Slag phase
2. Chromium Production:
- Chromite ore+Coke→Electric furnace reduction→Chromium-iron alloy
- Byproduct slag containing 5-15% Cr₂O₃
Typical Composition Comparison(%)
| Component | Vanadium Slag | Chromium Slag |
| V₂O₅ | 8-12 | <1 |
| Cr₂O₃ | 3-8 | 10-18 |
| Fe₂O₃ | 35-45 | 25-35 |
Regional Distribution:
- Panxi Region: Primarily vanadium slag
- Shanxi/Inner Mongolia: Chromium slag
- Coastal Steel Mills: Mixed types
What's the Current Status of Vanadium-Chromium Slag?
Mountains of vanadium-chromium slag nationwide are creating “red alerts.” Panzhihua City alone has accumulated over 30 million tons, covering 200 standard football fields.
China’s comprehensive utilization rate remains below 30%, with over 70% still stockpiled. This highly alkaline waste (pH 11-13) contains hexavalent chromium concentrations 2,000 times the drinking water standards. Rainfall can cause severe groundwater contamination, yet each ton contains ¥3,000-5,000 worth of recoverable vanadium.
How to Process Vanadium-Chromium Slag?
Every ton of untreated vanadium-chromium slag poses environmental risks equivalent to 500kg of cyanide. But with proper processing, this “industrial poison” can become a goldmine of strategic metals.

Traditional Vanadium-Chromium Slag Treatment Methods
Traditional vanadium-chromium slag treatment primarily relies on stockpiling and blast furnace detoxification, but these methods present significant environmental and resource efficiency issues:
Stockpiling Method
Land Occupancy: Large volumes of vanadium-chromium slag accumulate, occupying production land;
Secondary Pollution Risks: Long-term open-air storage may cause heavy metals (vanadium, chromium) to leach into soil and groundwater via rainwater, or contribute to air pollution through windborne dust.
Blast Furnace Detoxification Method
High Energy Consumption: Requires reincorporating slag into sintered ore for high-temperature reduction and solidification, consuming substantial amounts of coke.
Environmental Hazards: Dust dispersion or leachate leakage risks during transportation and smelting.
Resource Waste: Valuable metals like vanadium and chromium are not effectively recovered, instead being directly solidified into ironmaking byproducts, losing economic value.
Industry Pain Points Summary: While traditional methods achieve short-term harmless disposal, they fail to balance environmental friendliness, energy efficiency, and resource recycling. Innovative technologies are urgently needed to break this impasse.
Current Status of Vanadium-Chromium Slag Resource Utilization Technologies
As the metallurgical industry places increasing emphasis on environmental protection and resource recycling, the resource recovery of vanadium-chromium slag has gained significant attention. The current mainstream technologies include roasting-leaching, direct acid leaching, reduction smelting, and alkaline oxidation, each exhibiting notable differences in recovery efficiency, cost, and environmental feasibility.
1. Roasting-Leaching Method
Principle: High-temperature roasting modifies the phase composition of vanadium-chromium slag, followed by acid/alkali leaching to extract valuable metals.
Variations
- Blank Roasting: Direct roasting followed by water leaching, achieving high vanadium recovery but poor chromium extraction.
- Sodium/Calcium Roasting: Addition of Na₂CO₃ or CaO improves vanadium conversion rate (>85%).
- Chromate Roasting: Oxidative roasting converts Cr³⁺ into water-soluble Cr⁶⁺, but poses hexavalent chromium pollution risks.
- Sulfation Roasting: Sulfuric acid reacts to form soluble sulfates, enabling high co-extraction of vanadium and chromium.
Pros & Cons
- Mature technology, suitable for large-scale operations;
- High energy consumption, some processes emit toxic gases (SO₂, Cl₂).
2. Direct Acid Leaching
Principle: Uses sulfuric acid or hydrochloric acid to directly dissolve vanadium and chromium without prior roasting.
Key Features
- Typically enhanced with oxidants (H₂O₂, NaClO₃) to convert Cr³⁺ into Cr⁶⁺, improving leaching rates.
- Achieves 80%–90% vanadium recovery and 60%–75% chromium recovery.
Pros & Cons
Short process flow, low energy consumption;
- High acid consumption, wastewater contains heavy metals and residual acids, increasing treatment costs.
3. Reduction Smelting Method
Principle: High-temperature reduction smelting separates vanadium-chromium alloys from slag.
Application Scenarios
- Suitable for high-chromium slag (Cr₂O₃ >10%), with vanadium and chromium enriched in ferroalloys.
- Vanadium recovery >85%, chromium recovery >90%.
Pros & Cons
- High resource utilization, alloy products directly reusable;
- Requires advanced equipment (electric/plasma furnaces), high operational costs.
4. Alkaline Oxidation Method
Principle: Alkaline leaching (e.g., NaOH/Na₂CO₃) combined with oxidizing conditions (e.g., pressurized alkali leaching).
Advantages
- Selective dissolution of vanadium, leaving chromium in residues for separate recovery.
- Environmentally friendly, with no acidic wastewater or exhaust gases.
Pros & Cons
- Sustainable process, ideal for high-alkalinity slag;
- Slow reaction kinetics, alkaline solution recovery is challenging.
Comparative Analysis & Technology Selection
| Technology | V Recovery | Cr Recovery | Energy Use | Eco-Friendliness | Best Applications |
| Roasting-Leaching | 70%–90% | 50%–80% | High | Medium (gas emissions) | Large-scale steel plants |
| Direct Acid Leaching | 80%–95% | 60%–75% | Low | Poor (wastewater issues) | Small-scale plants, low-Cr slag |
| Reduction Smelting | >85% | >90% | Very High | Medium (slag harmless) | High-chromium slag, alloy production |
| Alkaline Oxidation | 75%–88% | <50% | Medium | Excellent | Eco-sensitive regions, V-first recovery |
Future Directions
- Developing low-temperature catalytic roastingor bioleaching technologies to reduce carbon footprint.
- Optimizing multi-stage extractionfor deeper vanadium-chromium separation.
- Advancing acid/alkali recyclingto cut overall costs.
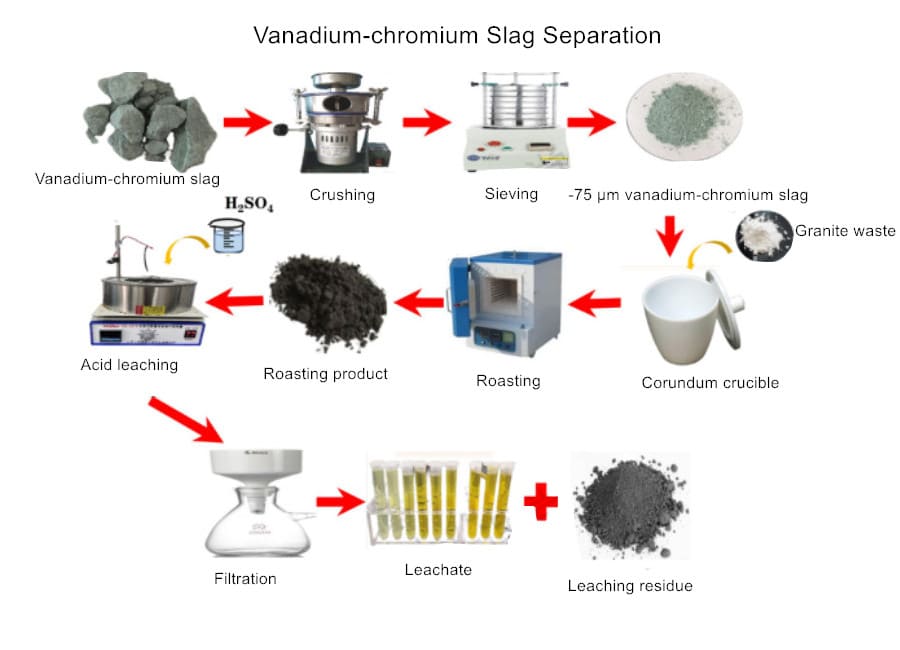
Vanadium-chromium slag processing involves several stages, including crushing, roasting, leaching, and purification, to recover valuable metals. Modern methods achieve over 90% vanadium and 80% chromium recovery rates while neutralizing toxicity.
Conclusion
Vanadium-chromium slag represents both an environmental challenge and an opportunity for sustainable resource recovery. While traditional methods, such as stockpiling and blast furnace detoxification, remain common, newer technologies—such as roasting-leaching, direct acid leaching, reduction smelting, and alkaline oxidation—offer more efficient and eco-friendly alternatives. The future lies in optimizing these processes to maximize metal recovery while reducing energy consumption and waste generation. As industries move toward circular economy models, improving vanadium-chromium slag utilization will be crucial for balancing economic growth with environmental responsibility.